Helping businesses keep electronic records and gain approval for critical transactions based on US FDA requirements
In March 1997, the U.S. Food and Drug Administration (FDA) issued final Part 11 regulations for the acceptance of electronic signatures under certain circumstances. Under this rule, the FDA approved e-signatures-and-e-records, making them as valid as paper records and traditional handwritten signatures. After this revolutionary decision, many industries, including pharmaceuticals, food, medical devices, and biotech started adopting this modern technology.
Traditionally, many life science companies struggled to manage massive amounts of data on paper. As a result, they experienced inefficiencies in their operations, productivity, product quality, and compliance processes. In this COVID era, even more industries are moving to electronic business management systems that enable less time-consuming data validations and more compliance with regulatory authorities.
Oracle Cloud technology provides E-Signatures and E-Records (for maintaining electronic signatures) with strict role-based secure transactions and validations. They can be used for different business events in Oracle Fusion Inventory Management, Manufacturing, and Quality inspections. As per FDA provisions, the following controls and requirements should be fulfilled for e-signatures:
- Limiting system access to authorized individuals
- Using authority checks
- Using device checks
- Recording changes in a manner that does not obscure earlier entries
- Using secure computer-generated date and time stamp to record the operator entries and actions
- Using operational sequence checks to enforce sequencing records
The 1997 FDA rule applies to all areas of Title 21 of the Code of Federal Regulation (CFR) for manufactured drugs and medical products distributed by pharmaceutical, biotechnology, and medical device companies in the United States. It authorizes e-signatures for their manufacturing, maintenance, and laboratory records. Oracle has built E-Signature and E-Records functionality for most of the business events related to these processes.

Considering E-Signatures and E-Records?
E-Signatures and E-Records are easy to use and do not require rigorous training. Your implementation team can address approval hierarchy, approval rules, business process flows, and more. In addition, with E-Signature and E-Records:
- Accurate, customized authorizations are available to manage critical business transactions.
- All edits, versions, additions, and updates are visible and documented per regulatory requirements.
- Audits trails can be downloaded and shared at any point in time.
- All mandated regulatory requirements for supply authorization are already embedded in the current system.
- There are no limitations on digital records storage, so you do not have to worry about removing old records for the sake of saving space.
Here are some of the solution-specific considerations to keep in mind regarding E-Signatures and E-Records:
Oracle Fusion Manufacturing: Manufacturing industries including pharmaceuticals, food, beverages, medical devices, and biotechnology use E-Signature and E-Records for their regulatory compliance initiatives, to reduce paper-based documentation, and to enable transactions such as bills of material creation, routing definitions (operation management), manufacturing order transactions, etc.
Oracle Fusion Inventory Management: E-Signature and E-Records can be leveraged when authorized signatories are needed and for business events including miscellaneous transactions, inventory receipt and delivery, and lot/serial updates.
Oracle Fusion Quality Inspection: You can assign authorized individuals to verify and approve quality inspections. As per regulatory requirements, quality inspection data needs to be stored and validated for all kinds of manufacturing products. This E-Signature and E-Records functionality provide compliance with regulatory authorities.
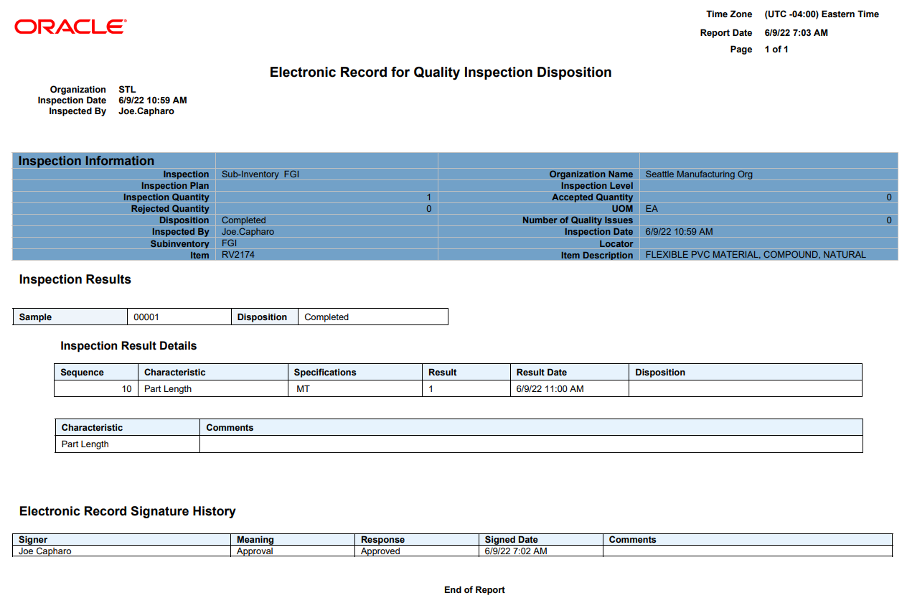
Keep in mind, too, that Oracle Cloud already provides standard business events to enable E-Signature and E-Records. Businesses can identify, based on their specific regulatory requirements, which events need to be considered during implementation.
It’s also worth noting that there are two types of process flows in Oracle Fusion:
- Inline approval process: In this process, approval must be obtained before the transaction is saved. If the transaction is rejected, then it cannot be saved.
- Deferred approval: This transaction is saved in pending approval status before initiating E-Signature processes. Once approved its status changes accordingly.
Inspirage can help
Businesses in industries ranging from pharmaceuticals to food to biotech can benefit from embedded E-Signature and E-Records functionality, which helps to cut costs, increase connectivity between departments, enhance operational efficiency, and improve product quality. Inspirage understands the challenges you face and can help your business successfully implement E-Signature and E-Records as well as integrate any existing third-party e-signature software you may already have. Please contact us to learn more.
As the Integrated Supply Chain Specialists, with recognition from Gartner, IDC, and winners of Oracle’s 2021 Game Changer Award for SCM Service Delivery Partner of the Year, Inspirage is uniquely qualified to be your success partner. Whether you are upgrading your on-prem system or have decided to move to the cloud where continuous improvement is built-in, our team is prepared to guide you on your transformational journey.
